By Josh Cosford, Contributing Editor
Physical laws, unlike governmental laws, are perfect. The heavy hand of the (physical) law keeps our fluid power realm in check, dictating liquids and gases’ behaviours at every location. I’d like to say very few alternate engineering fields so heavily rely on these laws and principles as fluid power, but a few may be too large a number. In fact, I submit that fluid power engineers and designers cogitate these laws more than engineers within any other vocation.
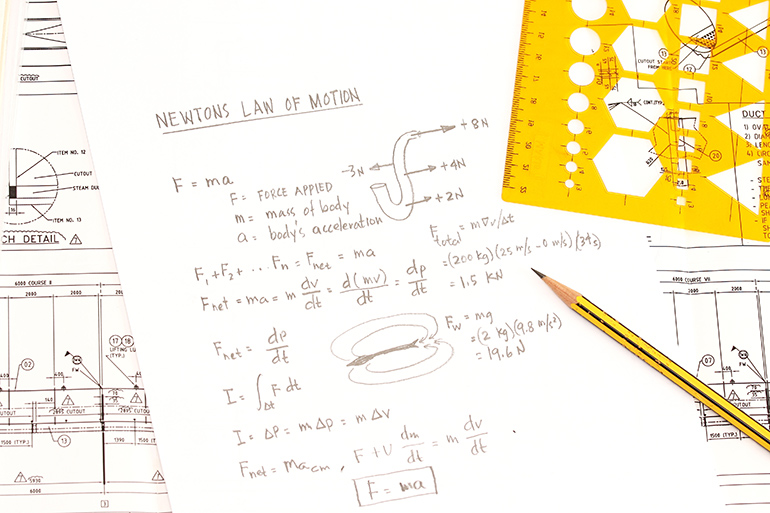
I shall not make such a claim with no body of evidence at my back, so here I list the laws and principles most relevant to fluid power. Some laws loosely apply and are widely referenced, such as Newton’s Laws. Others, such as the gas laws, are profoundly abundant in pneumatics. At any rate, you may be surprised just how many laws rule the fluid power realm.
Pascal’s Law
Pressure applied to a fluid in a confined chamber is transmitted undiminished to every area without loss and at right angles to each surface. This law is why fluid power is what it is. We pressurize liquids and gases and then transmit that pressurized fluid in a controlled manner to achieve work.
Newton’s Laws of Motion
- First Law of Motion — a body at rest tends to stay at rest, and a body in motion tends to stay in motion unless acted upon by an outside force. In fluid power, that body is ultimately the load moved by the actuator. I should mention that even air and hydraulic fluid must be first acted upon by a compressor or pump before acting upon the actuators.
- Second Law of Motion — an object’s rate of velocity change is proportional to the magnitude of force applied to it, expressed in the equation force = mass x acceleration. Although many hydraulic designers use flow to calculate the hydraulic actuation rate, motion control applications require advanced math using the VCCM equation, which factors force not flow.
- Newton’s Third Law of Motion — every action has an equal and opposite reaction. This one partially explains Cosford’s Law (see below), in that restrictions only create pressure because they are an opposing force.
Gas Laws
The gas laws comprise many individual laws first observed by just as many individuals observing effects on the volume, temperature, pressure, mass and constants under various conditions. Although these laws were observed over a period spanning well over a century, their combined use allows us to understand what happens when we compress, expand and cool air in pneumatic systems. Here are the relevant laws to fluid power.
- Boyles’ Law — volume of a given mass of gas varies inversely proportional to a gas’s pressure, so long as the temperature remains constant.
- Charles’ Law — temperature and volume of a given gas will vary in direct proportion to each other, so long as pressure remains constant.
- Gay-Lussac’s Law — pressure and temperature of a given gas will vary in direct proportion to each other, so long as volume remains constant.
- Ideal Gas Law — a combination of many other gas laws put into a single equation. It states that the factors of pressure and volume are equal to the factors of temperature, amount of gas molecules and the ideal gas constant. The ideal gas law is a popular method to calculate the effects on air as it is compressed for the pneumatic system, or the relationship between pressure and temperature when using hydro-pneumatic accumulators.
Laws of Thermodynamics
- First Law of Thermodynamics — also called the Law of Conservation of Energy, states that total energy in a closed system remains constant. Energy can neither be created nor destroyed but can only change forms. You absolutely must understand this when designing fluid power systems. The energy at your actuators can never exceed your prime mover’s energy capacity, and the differential between input and output exists as waste energy in various forms.
- Second Law of Thermodynamics — separate systems, when combined, will move to a state of equilibrium. Energy can only move from an area of higher potential to a place of lower potential. When provided a downstream path of reduced pressure, a charged accumulator can only exert its energy in that downstream direction. The lower downstream pressure will never continue to lower itself as energy moves to the higher state inside the accumulator.
The Second Law also explains the myth of “pressure is resistance to flow.” A restriction that results in a pressure increase downstream of the pump is not what created the pressure. Pressure absolutely had to start at the pump and made its way to the restriction before Newton’s Third Law of Motion explains how the restriction simply “pushes back” to increase pressure everywhere between itself and the pump.
- Third Law of Thermodynamics — energy in a closed system can only evolve into a state of increased entropy. Entropy is a state of disorganization and explains how energy added to your fluid power machine has finite purpose and will decay at every step of the way from input to actuator. Your actuator will never produce more power than an air compressor or hydraulic power unit supplying it.
Cosford’s Law
I haven’t discussed Cosford’s Law in a couple of years, but it’s due time. I created Cosford’s Law not to see my name in print but as a quick phrase to remind everyone of hydraulic energy’s true nature. The proliferation of the “flow makes it go” mentality required a counterstatement. The law states that “pressure makes it go; flow is the rate in which you can create pressure.”
Riding on the coattails of both Newton’s Third Law and the Second Law of Thermodynamics, Cosford’s Law tells us flow only occurs because of pressure, not the other way around. Pressure is force over a defined area, and it’s the force that gets the fluid moving, not the other way around. Force pushing on oil molecules is no different from pushing on a solid steel rod, except that with oil, the molecules move with fluidity relative to each other.






Thanks for the educational posting. And I appreciate Cosfords Law as a way to keep discussions honest.
All of the gas laws can be summarized as PV=nRT, the universal gas law.
And thermodynamics is an excellent reminder that nothing just happens, everything is started by the application of energy. Energy being applied or removed is the cause of all motion and temperature change, which can admittedly be difficult to see or measure. But just because I fail to see something does not mean that it is not there, or did not happen. But that is an entirely different discussion.